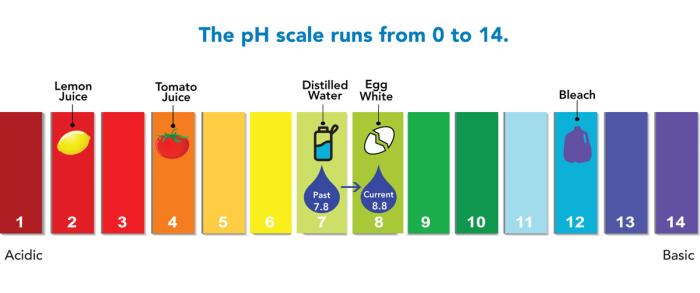
Hitting a target of 8.8 on the pH scale
Editor’s note: This is part three of a TAP series about the work that went into raising and maintaining the pH of the water Denver Water delivers as part of the utility’s groundbreaking Lead Reduction Program.
The series will continue over coming weeks. Click here to read part one. Sign up here to receive our free weekly TAP newsletter.
As January 2020 started, the clock was ticking.
In the last weeks of 2019, state and federal health officials had agreed to Denver Water’s multipart proposal to reduce the risk of lead from old, customer-owned lead service lines getting into drinking water.
With approvals in hand from the Environmental Protection Agency and the Colorado Department of Public Health and Environment, Denver Water’s Lead Reduction Program launched in the first days of the new year.
The program involved locating and replacing between 64,000 and 84,000 customer-owned lead service lines usually found in Denver-area homes built before 1951, distributing more than 100,000 pitchers and water filters certified to remove lead from drinking water, and launching a major public health education program.
Read more about Denver Water’s groundbreaking Lead Reduction Program.
But the biggest part of the new, groundbreaking program was raising the pH level of the water Denver Water delivered across more than 3,000 miles of pipe to 1.5 million people. The deadline to for the change was before the end of March — less than 90 days away.
And Denver Water’s people were ready.
Engineers, operators and others throughout the utility had been hard at work during the previous few years. Not knowing if state and federal regulators would approve a pH change, Denver Water had prepared its drinking water treatment plants and staff to implement either the addition of orthophosphate or a higher pH level.
“We’d been planning for two different futures, either orthophosphate or pH. Everything we’d been doing, planning and designing for was for one of those two possible futures,” said Ryan Walsh, a water treatment engineer.
Walsh’s team was in charge of testing various treatment options via a pipe loop study that had been underway for years (and still continues today) and later planned, designed and executed the treatment plant systems involved in increasing the pH level.
Now Denver Water knew which path to focus on: adjusting the pH from 7.8 to a new target of 8.8 by mixing in sodium hydroxide, a common water treatment technique the utility had been using since the 1990s to raise the pH of the water it delivers and reduce its corrosiveness.
And not only would the utility simply raise the water’s pH level to the new target.
Denver Water also laid down a new challenge to its staff: Raise the pH level to 8.8 and keep it there, without much wiggle room, across the utility’s 3,000 miles of pipe — so that the pH level of the water was still at 8.8 or very close to that level when the water arrived at customers’ homes and businesses.
For the pH target of 8.8, the utility’s extremely small variance is just plus or minus 0.2 for water leaving the three drinking water treatment plants, and only plus or minus 0.3 for water across the 3,000 miles of pipe in the delivery system.
Raising the pH level of drinking water above a certain point isn’t unusual in the water industry. Maintaining the pH level in such a narrow band is a rarity.
“This was a big change in how we not only managed the pH, but how we ran our pumps, analyzers and controls. We now were looking not only the pH in the plant but the stability of the pH downstream in the distribution system," said Patty Brubaker, a water treatment plant manager.
"At the time it was very stressful trying to keep the pH in such a tight range. It took a lot of teamwork and coordination from the source water supply teams, to the treatment plants, to the miles of distribution piping,” she said.
Watch this video with Nicole Poncelet-Johnson, head of water quality and treatment, discussing how pH fits into the Lead Reduction Program.
“The protective coating we are now encouraging to form inside the lead pipe with the pH at 8.8 is a stronger version of what we had at 7.8,” said Nicole Poncelet-Johnson, the head of water quality and treatment at Denver Water.
But the pH has to stay at that level, day after day, month after month, year after year, in order to keep that protective coating strong.
“If you have wide variations in the pH level of the water flowing through the old lead service lines, you can create a softer layer in that coating, like in the snowpack — when there’s a softer, weaker layer of snow, it can cause an avalanche,” Poncelet-Johnson said.
Learn more about this TAP series on pH, the biggest part of the Lead Reduction Program.
“We want to maintain that 8.8 pH level, and stay in that tight range around 8.8, not just at the plants but all across the entire distribution system.”
The new challenge drove work groups together.
“Engineers are really good at designing things. And operators are really good at optimizing the treatment plants to produce safe, high quality drinking water. We’d all worked together in the past, but the new focus on the pH level took that collaboration to a new level,” said Walsh.
Meetings grew to 20-person conference calls as experts from different Denver Water divisions hashed through various factors that could affect pH levels — from the weather in the mountains as the water flowed through reservoirs and down streams to the treatment plants, to the soil temperatures around miles of delivery pipes buried in the streets.
They discussed how the utility’s teams could counter those factors to keep the pH stable from the treatment plant to the customer’s home and what changes were needed at Denver Water’s three drinking water treatment plants, where the pH level is raised.
Denver Water has used sodium hydroxide to adjust the pH of the water it delivers since the 1990s. Raising the pH levels from 7.8 to 8.8 required adding storage to the treatment plants and making physical and programming improvements to chemical feed systems. Equipment inside the plants needed to be adjusted or added to ensure the pH is raised to the proper level.
Engineers worked with plant operators to design systems that worked and also were easy to use. New operating protocols were established that were easy to follow and ensured the new pH target was hit as millions of gallons of water flowed from the plant into Denver Water’s distribution system.
“Working through this really gave each of us a systemwide view. We realized we all have an impact on each other,” said Russ Plakke, who leads a team that focuses on improving operations at Denver Water’s treatment plants and troubleshooting issues that rise.
As engineers and treatment plant operators worked to finetune the equipment inside the plants, Denver Water’s water quality field teams also were preparing.
Their job is monitoring the quality of the water as it flows from mountain to tap, collecting water samples and checking its temperature, clarity, conductivity and pH level.
And — just as with other teams across Denver Water — the utility’s increased attention on pH levels was about to change their jobs too.
Stay tuned to TAP to learn how Denver Water’s field teams adjusted to the increased focus on pH in the fourth installment of our pH series, set to drop in the coming weeks.
If you missed earlier parts of this series, click here to read part one and find links to the rest.