Denver Water collects snow and rain that falls in the mountains to supply 1.5 million people with water in the metro area.
While water that flows through mountain streams looks crystal clear, it must go through a treatment process to make it clean and safe to drink.
Denver Water has three treatment plants that take mountain water and purify it for our customers.
In short summary, the treatment process is used to remove unwanted material such as dirt, sand, sediment, contaminants, metals, parasites, viruses and bacteria from the water while giving water an enjoyable taste and smell.
6 Steps of Water Treatment
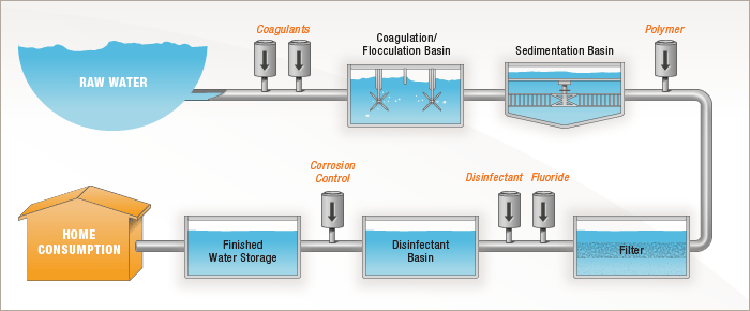
Coagulation
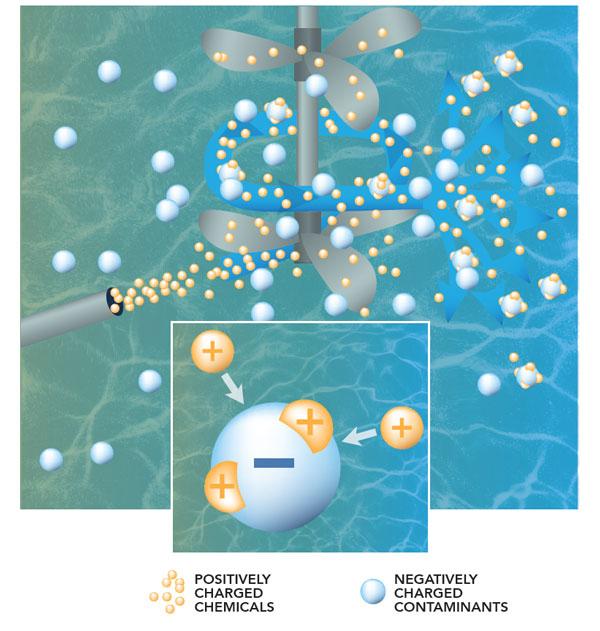
The treatment process begins as water from the mountains is brought into a tank at the treatment plant. At this point, the water contains particles of sediment and other unwanted material that must be removed.
The microscopic particles are so tiny that they will not settle out on their own, so we use a process called coagulation to join them together so it’s possible to remove them.
Because the particles have negative charges, they repel each other like magnets. To solve this problem, coagulants, like aluminum sulfate that have positive charges, are added to the water. This causes a chemical reaction which neutralizes the negative charges, so the particles are able to join together (think opposites attract.)
Flocculation
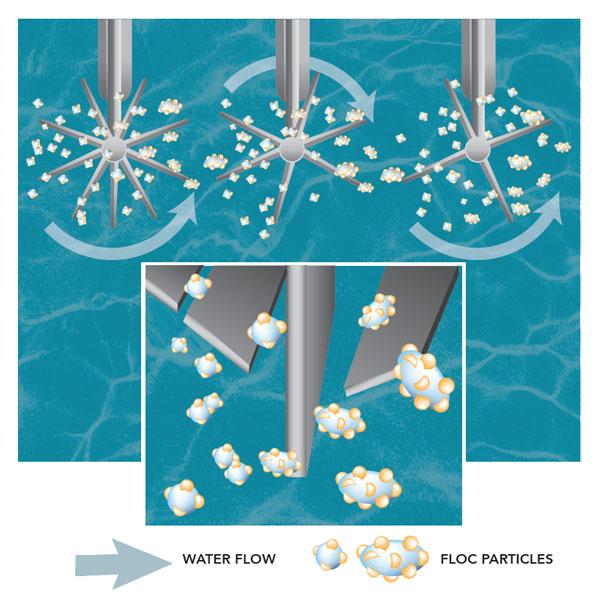
As the negatively charged particles and the positively charged coagulants are joined together, they form larger particles called floc.
Flocculation is the process of gently stirring the water so smaller floc particles can bump into each other and join together. Floc particles continue to collide and grow larger and heavier throughout this process.
Sedimentation
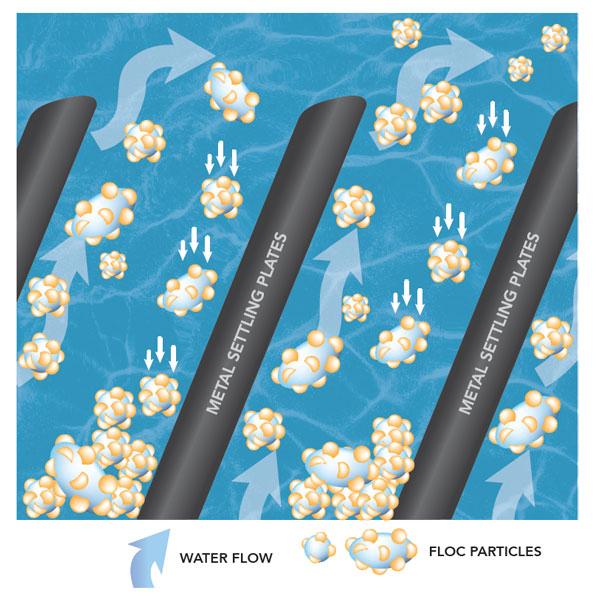
The result of coagulation and flocculation comes to fruition during the sedimentation process. This is where the clumps of floc are now heavy enough to fall to the bottom of a tank. The sludge is removed and the water moves through to another tank.
The water is much cleaner after this process, but there are still some remaining particles in the water.
Filtration
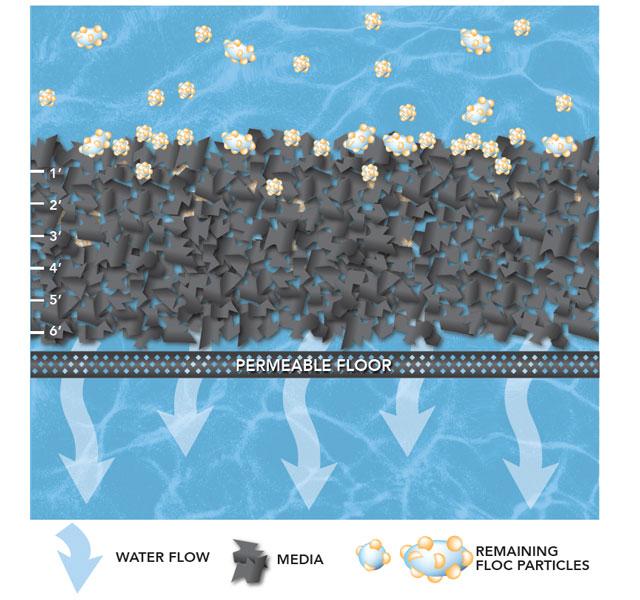
Filtration is where the tiniest particles are removed from the water. The filtration process happens inside a tank that contains a layer of anthracite coal and a layer of sand which are known as “filter media.”
As the water moves through the filter media, larger particles get caught in the spaces between the grains of anthracite.
Water passes through the filter media and exits through pipes at the bottom of the tank. Water that leaves the filter beds is crystal clear.
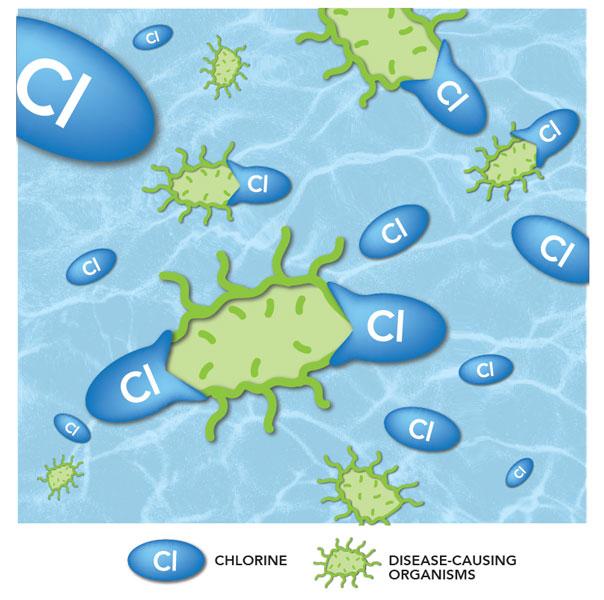
Disinfection
Now that all the sediment has been removed, the water moves to the disinfection process inside another tank.
In this step, we add disinfectants such as chlorine to the water. The disinfectants attack any disease-causing organisms (bacteria, viruses and parasites) that may be in the water and destroys it.
The clean water flows through a series of baffles to provide enough contact time with the disinfectants. This process also protects the water as it moves through pipes in the city.
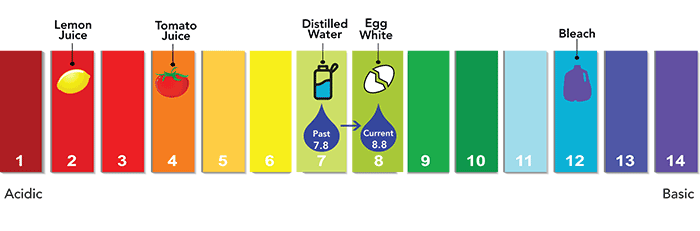
Corrosion control
A final step in the process involves corrosion control. While there is no lead in Denver Water, for decades, the utility has taken steps to protect treated clean water from customer-owned lead service lines and lead plumbing. To do this, we raise the pH level of the water by adding caustic soda.
This step is an industry standard practice and reinforces the protective coating inside lead pipes to prevent lead particles from leaching into the water if a customer has a lead service line or lead in their plumbing. The pH level was raised in 2020 as part of Denver Water’s Lead Reduction Program.
Want to know more about how we get clean, high-quality water to your tap? Join an animated tour of the potable water treatment process.