Need more details about your water?
Your water’s journey starts as mountain snowpack and ends with the clean, great-tasting water that comes out of your tap. Last year, we collected more than 55,000 samples and conducted nearly 200,000 tests to ensure our water is as clean and safe as possible.
Some customers, such as beer brewers, pet stores or coffee shops, have more detailed questions about water hardness, metals and compounds than what’s included in Denver Water’s annual water quality reports. Those compounds, including emerging concerns, are listed below.
Watershed and reservoirs
Denver’s drinking water comes from rivers, lakes, streams, reservoirs and springs fed by high-quality mountain snow runoff. Denver Water’s supply is 100% surface water that originates in sources throughout 3,100 square miles of watersheds on both sides of the Continental Divide.
Treatment
Denver Water’s three treatment plants remove particulate matter and microscopic organisms that are found in surface waters. After filtration, chloramines are added as a disinfectant to inactivate potentially harmful microorganisms. Chloramines provide a longer lasting disinfection method than free chlorine, keeping water clean throughout Denver’s extensive distribution system. They also produce lower concentrations of disinfection byproducts than free chlorine.
Denver Water’s water quality laboratory analyzes and tests samples before and after the treatment process for compliant and noncompliant compounds. Some compounds may not be regulated by state or federal agencies, but Denver Water monitors them. All drinking water, including bottled water, may reasonably be expected to contain at least small amounts of some contaminants. The presence of contaminants does not necessarily indicate that the water poses a health risk.
Emerging contaminants
Because Denver Water’s drinking water supply originates in high-elevation rivers and reservoirs, PFAS-related compounds have not been an area of concern in our source water. We do test quarterly and since we started testing for PFAS compounds in 2017, we have not detected any in our drinking water.
- Perfluorocarbons (PFCs) — Poly-fluorinated-carbons (PFCs) and poly and per-fluoroalkyl substances (PFAS) are found in food packaging, cookware, outdoor gear, furniture, carpeting and aqueous film forming foams (AFFF) used in firefighting. Denver Water monitors the influent and effluents of the potable treatment plants and has shown no detectable levels of PFCs in the drinking water .
- Cyanotoxins — Cyanobacteria, commonly known as blue-green algae, are a class of aquatic microorganisms that can produce cyanotoxins. Health effects include gastrointestinal discomfort, liver inflammation, and skin rashes or dermatitis. Climate change is causing these algae blooms to occur more often than they used to. Denver Water continuously monitors and samples its source water for the presence of cyanobacteria and nutrient concentrations that favor bloom occurrences. Denver Water can switch or augment its source water to optimize water quality. To date, Denver Water has not had any detectable levels of cyanobacteria in its source or distribution water.
- Microplastics — Plastic materials are found everywhere and are slow to degrade. Microplastics are characterized as plastic material smaller than 5 millimeters and larger than 10 nanometers, but most concerning are plastic particles smaller than 25µm (micrometers) because they can pass into human tissue, causing inflammation and digestive problems. Denver Water is working with other labs and water industry professionals to develop consistent, repeatable and widely available methods of detecting and quantifying plastics in drinking water.
- Taste and odor — During the height of runoff throughout summer and the rapid temperature changes of the fall, there may be instances in which water will have an unusual or unfamiliar taste and/or odor when dissolved organic compounds are released from the dynamic microscopic community of organisms (including diatoms, plankton, zooplankton, algae and cyanobacteria) that are present in source water. These compounds are not harmful for human consumption; however, Denver Water continuously monitors and adjusts its source water to minimize potential taste and odor events. Denver Water can add powder activated carbon (PAC) into the treatment process to remove these compounds to make drinking water more palatable.
- Lead — The water that Denver Water provides to homes and businesses is lead-free, but lead can get into the water as it moves through lead-containing household fixtures, plumbing and water service lines — the pipe that brings water into the home from the main in the street — that are owned by the customer. Denver Water is underway with its Lead Reduction Program, a 15-year effort to locate and replace the estimated 64,000 to 84,000 lead service lines in our service area.
- Pharmaceuticals – These compounds enter water systems through trace amounts passing through consumers as well as disposal of medications into sinks or toilets. Denver Water has monitored for these compounds in its source water, particularly areas that may be influenced by treated wastewater and agriculture.
Help us keep your drinking water clean
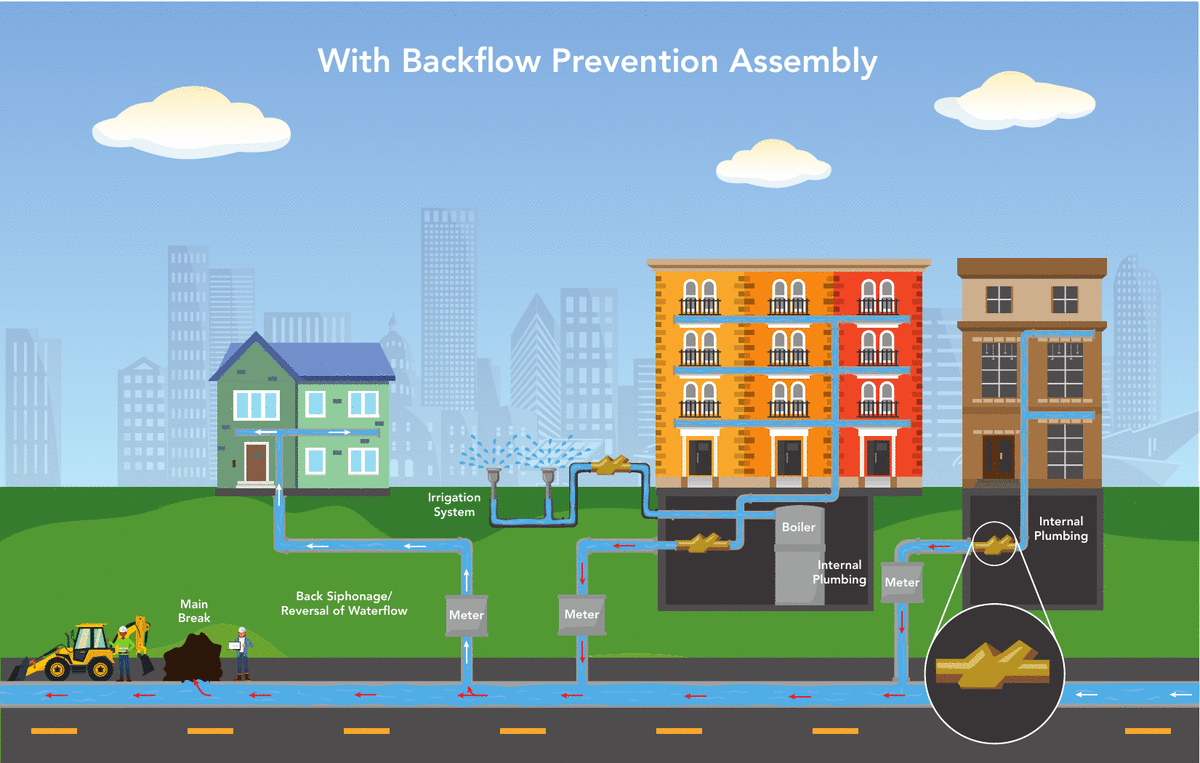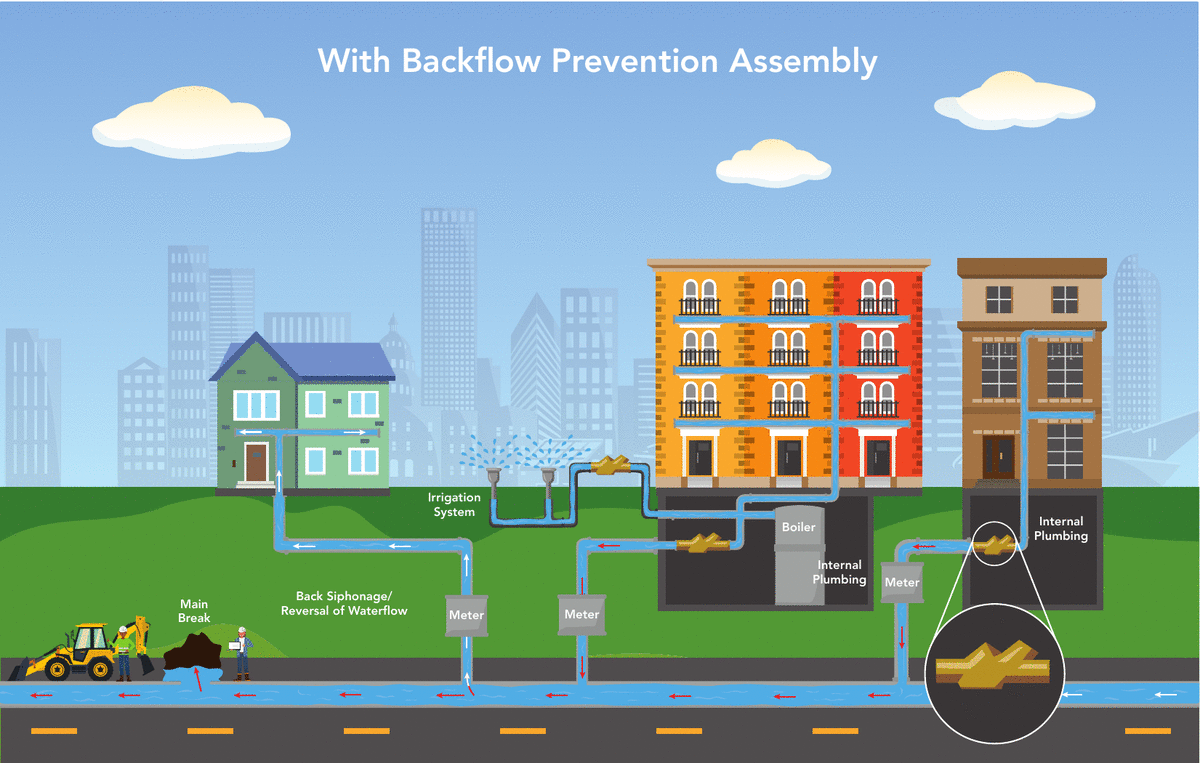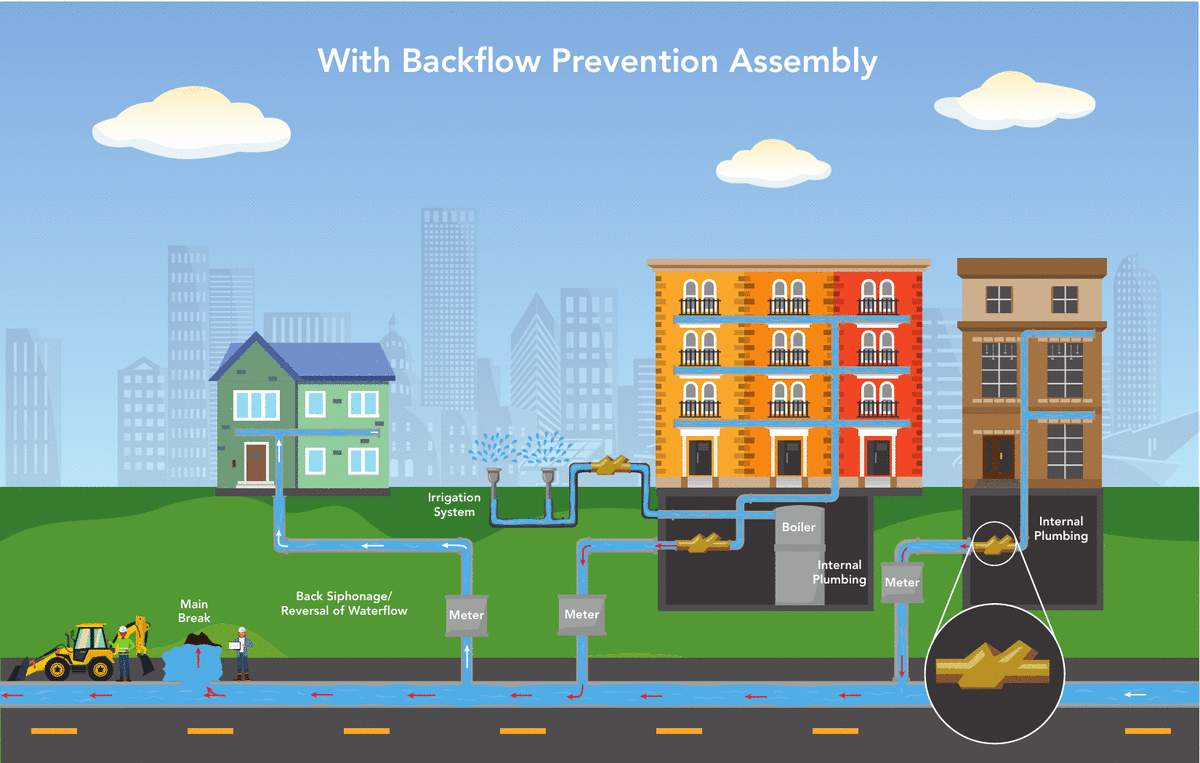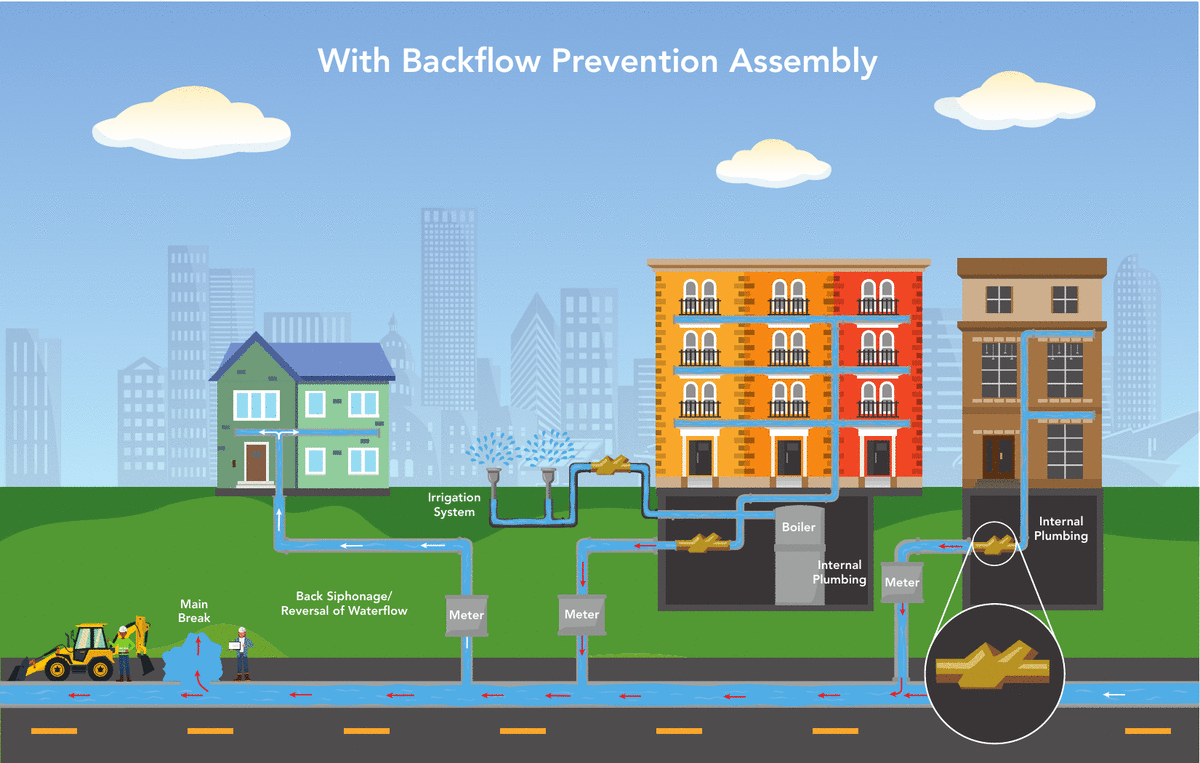
Denver Water’s Cross-Connection Control and Backflow Prevention Program protects the public water supply from pollutants and contaminants that could, under certain circumstances, be drawn into the public water supply from private properties. All commercial, industrial, domestic, irrigation and fire line services are required to have an approved backflow prevention assembly installed.
A backflow prevention assembly installed on the service line allows water to only flow into the building, preventing water from flowing in the opposite direction into the drinking water system. The sole purpose of a backflow prevention assembly is to prevent your drinking water from becoming contaminated.
What causes a backflow event?
Backflow is the unwanted flow of water or other liquids, mixtures, gasses or substances into the drinking water supply.
There are two main ways backflow can occur:
- Backsiphonage occurs when there is a negative pressure in the water distribution system, which draws the water from a private water system into the public water system. This can occur at any time, such as during a water main break or during a large firefighting effort.
- Backpressure occurs when the pressure in a private water system exceeds the pressure in the public water distribution lines that can cause normal flow to reverse. A pump used to increase the water pressure within a building’s plumbing system to reach a higher floor might cause this.
Click image to see it larger.
Click image to see it larger.
Why is a survey/test/installation necessary now, but was not required before?
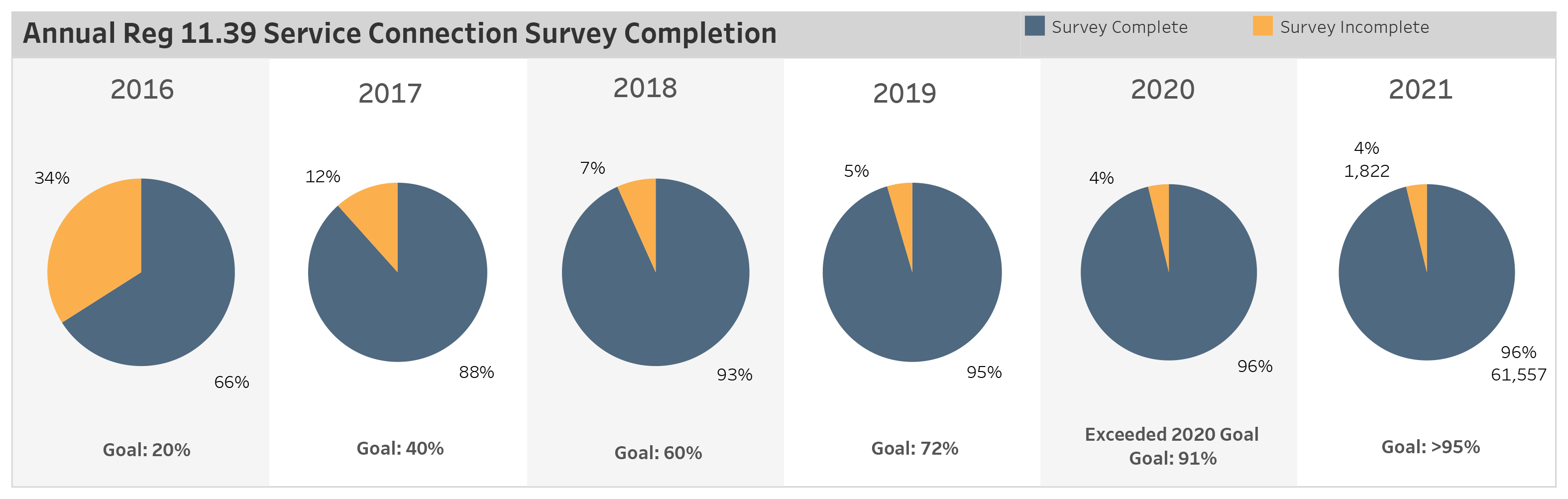
In 2016, the state passed State Regulation 11.39, mandating water utilities provide and maintain a cross-connection control and backflow prevention program. Water purveyors are required by the Colorado Department of Public Health and Environment to survey all water services to determine if potential hazards to the potable water supply are protected by a backflow prevention assembly.
Click image to see it larger.
What happens when Denver Water inspects my water service line?
A Denver Water employee will locate the water service line connection to the building. Then the employee will document the property type and any potential hazards, such as pesticides, chemicals, nonpotable water and others, to the water distribution system.
Backflow prevention assemblies contain the hazard from the potable water distribution system and are required to be tested annually. Denver Water processes more than 40,000 cross-connection control backflow test reports annually and reports the tests to the Colorado Department of Public Health and Environment to ensure the safety of your drinking water.
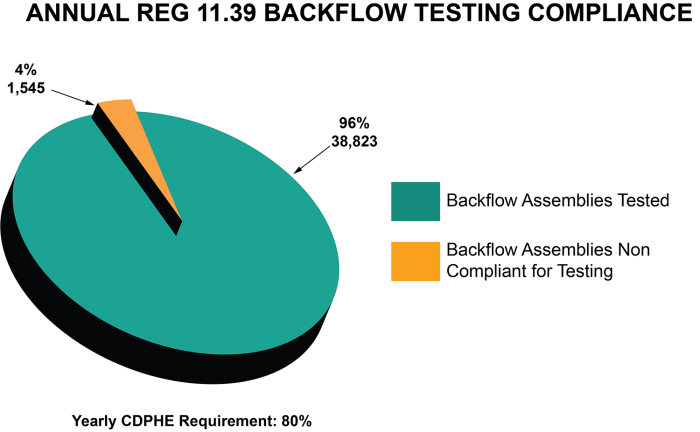
Backflow testing compliance
The chart below shows the number of backflow assemblies in Denver’s service area that complied with the state regulation annual testing requirements in 2021. Denver Water completes more than 60,000 surveys of backflow assemblies to comply with state regulations.